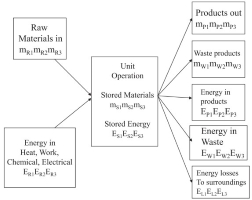
mass and energy balances
Material and energy balances are based on a conservation law which is stated generally in the form:
Change in total energy in the system = Total energy entering the system (Input) – Total energy leaving the system (Output)
Energy is the total energy of a system. The total energy of a system can be in the form of kinetic energy, potential energy, heat energy, and so on. These forms of energy can be in the form of other forms of energy so that the total energy in a system will always be the same.
Examples of Mass and Energy Equilibrium in Chemical Processes
Motor Vehicle
Chemical energy in the fuel is converted into kinetic energy in the engine. The amount of chemical energy contained in the fuel can not all turn into kinetic energy. Most of the energy that does not turn into kinetic energy will turn into other energy such as heat, friction, vibration , etc.
Energy other than kinetic that arises from the combustion process in the engine is caused by piston friction, friction in the gears on the gearbox, exhaust gases, radiator heat, wheel friction, and others. The type of unneeded energy that appears in the process is called losses.
The formula can be written as:
Total Energy Change = [Chemical Energy of the fuel (Total Input)] – [Kinetic Energy of vehicle speed (Total Output) – Losses (friction, exhaust gases, heat, etc.)]
Boiler
The simple principle of the boiler is to heat the liquid fluid that passes through the boiler using heat energy to become hot gas fluid (steam). However, heat energy cannot convert all liquid fluids into vapor completely due to losses. Some of the heat energy will be wasted due to the influence of radiation from outside the boiler, incomplete combustion of part of the volume of fuel, heat loss due to air and fuel dew, etc.
The formula can be written as:
Total Energy Change = [Heat energy in fuel (Total Input)] – [Heat energy in steam (Output) – Losses (radiation from outside the boiler, incomplete combustion, moisture in air and fuel, fuel residue, etc.)]
Cellphone
Power on the cellphone comes from a lithium battery that contains chemical energy. When the cellphone is charged, electrical energy from the socket flows into the battery and is stored in the battery into chemical energy. When a cellphone is used, the chemical energy from the battery is converted into electricity to power the screen (light energy) and speakers (sound energy). However, not all electrical energy is fully utilized by cellphone. There is some heat coming out of the cellphone or from the cellphone cable that causes our cellphones to heat up when operating. These are called losses.
The formula can be written as:
Total Energy Change: [Electrical energy in the socket (input) + Chemical energy in the battery (input)] – [Light energy on the screen (output) + Sound energy in the speaker (output)] – [Losses (heat coming from the cellphone and cable)]
So, we can formulate every activity, especially chemical processes, simply with the formula for the law of conservation of energy. This formula makes it easier for us to analyze what energies occur in a process.
Contributor: Daris Arsyada



Leave a Reply
Want to join the discussion?Feel free to contribute!